Stem Cell Therapy for Chronic Ischemia: A New Path to Limb Salvage
A clinical and molecular case for tissue-level revascularization using ArtAssist and G-CSF in chronic ischemia
by DARWIN ETON MD, FACS, DFSVS, ORCID# 0000-0001-6732-1413
Chief Science and Medical Officer, Vasogenesis Inc, 32 Marshal St., Boston MA 02446 USA
Funding information: Warren H. and Clara Cole Foundation; Cures Within Reach; Department of Surgery University of Chicago
Table of Contents
Why Neovascularization Fails in Chronic Ischemia – And How to Fix it
A therapeutic stem cell-based strategy was tested that seeks to improve the kinetics of neovascularization (NV) in patients with chronic ischemia. It was designed to overcome hemodynamic and cellular obstacles to NV that arise during the evolution of arterial disease. https://doi.org/10.1002/term.3284
NV is the normal mechanism by which Nature improves blood flow into ischemic tissue caudal to arterial occlusive disease. Vascular biologists and hematologists have elucidated many of the mechanisms involved. These contributed to the development of our strategy.
The Two Pillars of Vascular Repair: Arteriogenesis and Angiogenesis
NV can be broken down into two processes.
Arteriogenesisis the growth in diameter and in number of collateral arteries. It is initiated by the increased shear stress on the endothelial cells lining the luminal surface of arterial branches (side streets) that network around an occluded large artery (main avenue).
Angiogenesis is the hypoxia induced growth of the arterioles and capillaries that deliver the oxygenated nutritive blood flow to cells within the ischemic tissue, and of the venules that clear the waste products of metabolism. Angiographically, arteriogenesis is imaged as “cork-screw” shaped collateral arteries, and angiogenesis appears a blush in the tissue. Both accelerate the contrast transit through the ischemic tissue.
Limb Salvage without Surgery in Istanbul: A Breakthrough in Compression Therapy
NV fails to occur effectively as a number of obstacles arise. Ischemia poisons the biosynthetic micro-environment by impairing delivery of oxygenated nutritive blood flow and clearance of the toxic metabolites.
As the local buffering capacity is exceeded, the resultant acidosis accelerates protein denaturation and alters cell membrane permeability. A shortcoming of strategies to date that were designed to promote NV is the failure to first improve the ischemic bioenvironment.
Moreover, these strategies did not address the severely attenuated endothelial shear stress needed to initiate endothelial cell activation leading to arteriogenesis. This is especially important in patients with multi-level arterial occlusive disease that severely attenuates collateral artery flow.
Adittionly, distressed cells in the ischemic tissue secrete important signaling proteins (e.g. SDF-1α) meant to be carried by the venous return to niches of progenitor cells throughout the body to recruit these salutary cells back to the activated endothelium.
The poor blood flow not only impairs signaling protein synthesis and dissemination, it also attenuates the delivery of any mobilized cells back to the target tissue. The resistance of the arterial occlusive disease simply results in blood flow diversion and cell deposition everywhere else but the ischemic tissue.
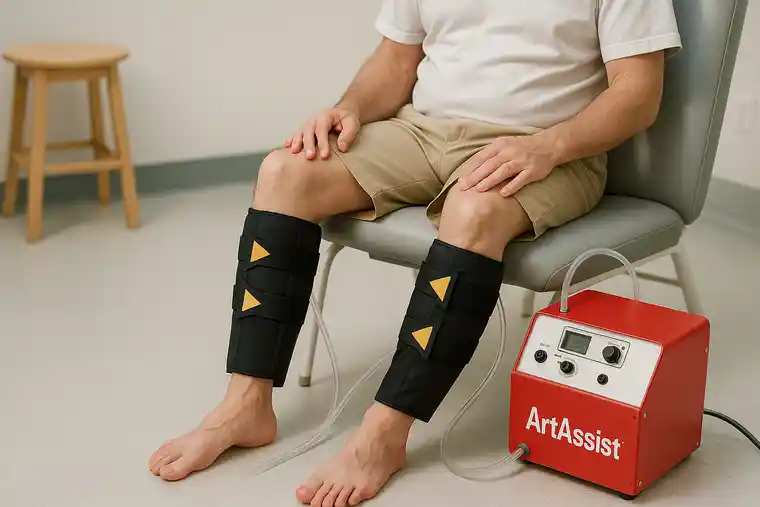
How the ArtAssist Device Supports Neovascularization
Improving oxygenated nutritive arterial inflow, clearing toxic metabolites, delivering the endothelial shear stress stimulus, and improving the dissemination and delivery of salutary proteins and cells all become increasingly important the further the ischemic tissue is from the heart. In patients with chronic limb-threatening ischemia (CLTI) we used a device that delivered a pulsatile mechanical force externally to the calf ankle and foot to address all these obstacles (ArtAssist device, ACI Medical (San Marcos, CA)).
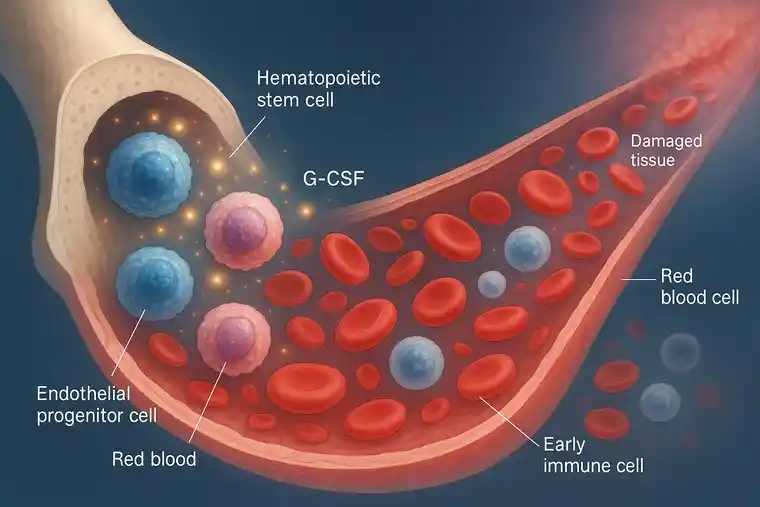
Stem Cell Mobilization with G-CSF: Boosting Blood Flow Naturally
NV is orchestrated in large part by cellular elements derived from the progenitor cell line and from inflammatory cells (like monocytes).
In patients with advanced age, diabetes, and severe vascular disease, a major obstacle to NV is the diminished number and function of circulating progenitor cells. As such, improving the biosynthetic micro-environment alone is not enough to improve NV, unless one can also improve the availability of the salutary progenitor cells and monocytes.
We elected to address this obstacle with Granulocyte Colony Stimulating Factor (G-CSF), commonly used in stem cell therapy to mobilize hematopoietic and progenitor cell mobilization and immune system amplification. We used Neupogen (amgen Inc) at a novel dosimetry (7-10 mcg/kg) every 72 hours for 10 doses in CLTI patients who wore the ArtAssist device daily for a minimum of 3 hours (while seated).
Limb Salvage Achieved in No-Option Patients
The CLTI patients treated were considered “no-option” CLTI after exhausting traditional revascularization options (surgical bypass, angioplasty, atherectomy, laser recannalization).
They were treated in lieu of major amputation. Limb salvage and healing of ischemic wounds in the first patients treated led to angiographic documentation of arteriogenesis, angiogenesis, and most surprisingly, evidence of recanalization of previously documented segmental arterial occlusion.
This led to the measure of plasma levels of plasmin (causes fibrinolysis) and of fibrin-degradation products (produced by thrombus undergoing fibrinolysis), along with a battery of pro-angiogenic proteins. These Enzyme Linked Immunosorbant assays (ELISA) confirmed that indeed our stem cell-supported approach led to NV, and the overcoming of another major obstacle to NV: chronic thrombus (https://doi.org/10.1002/term.3284).
Clinical and Molecular Evidence of Neovascularization
A detailed review of the cellular and molecular participants in NV and of their relevance to this promising NV strategy is available at https://doi.org/10.1016/j.jvsvi.2025.100190, which highlights how each of the cellular participants (endothelium, progenitor cells, neutrophils, monocytes, etc) contribute to explaining observations from the detailed molecular and cytometry assays we performed on our patients.
These data supported the hemodynamic and angiographic evidence of improved blood flow, and underscored how important it is to measure the molecular impact of a cell therapy (lacking in previous NV clinical trials).
“No-option” CLTI patients achieved limb salvage against severe odds. However, there is clearly a race between how fast our therapy works to improve blood flow versus the rate of deterioration from the advanced vascular disease.
While limb salvage is predicated on improved blood flow (the goal of our strategy), it is also dependent on advanced and diligent wound care, management of sepsis, debridement, nutrition, co-morbidities, compliance, and avoidance of trauma.
As such, implementing a NV strategy is likely best in the early stages of chronic ischemia, before any traditional interventions are done, and before ischemic wounds become so severe that sepsis requires extensive debridement. The more surgery is done, whether to revascularize with traditional means, or obligatory surgical wound management, the more altered the anatomy.
Acute inflammation and post-operative fibrosis are potential barriers to NV. A good example of this is that neutrophils in the setting of acute inflammation are pro-thrombotic and anti-angiogenic, whereas in the absence of acute inflammation neutrophils have fibrinolytic and pro-angiogenic capacity.
This context dependent activity of neutrophils is especially important in patients being treated with G-CSF, which causes a transient 5-12 fold increase in the absolute neutrophil count.
Why Traditional Interventions Fail in Chronic Ischemia
Traditional surgical and endovascular interventions are costly and invasive. Their benefit, when successful, is immediate restoration of flow.
Their weakness is durability, with intimal hyperplasia and recurrent disease being not infrequent causes of failure in the intermediate (6 months to 2 years) and long-term failure.
Importantly, acute correction of arterial flow is effective at alleviating symptoms but also arrests the intrinsic capacity for vascular repair (NV).
A Paradigm Shift Toward Tissue-Level Revascularization
The era has come to shift the focus in the management of chronic ischemia from acute large artery revascularization strategies to tissue level revascularization.
After all, no vascular surgeon was involved in the development and growth of our circulation (from a sperm and an ovum).
Nature knows how to do this, and indeed does it effectively until the obstacles discussed overcome the process.
Overcoming the obstacles restores NV, as seen with advanced stem cell and device-assisted therapies – offering durable, biologically aligned vascular repair.
This novel outpatient therapy costs a fraction of today’s interventions.

Dr. Darwin Eton
MD, FACS, DFSVS, retired Professor of Vascular Surgery
Honored for Advancing Research in the Management of Vascular Disease
About Dr. Darwin Eton
Dr. Darwin Eton, MD, FACS, DFSVS is a Retired Professor of Vascular Surgery and former director of advanced clinical programs in peripheral vascular disease. Over the course of his career, Dr. Eton has been instrumental in reshaping how vascular surgeons and scientists understand ischemic pathophysiology, particularly in the context of regenerative and cellular therapeutics.
To learn more about Dr. Eton’s academic and clinical contributions, refer to the news feature and search for:
“Darwin Eton MD, FACS Honored for Advancing Research in the Management of Vascular Disease”
If you wnt to go deeper in, please refer to:
A recent JVS article (https://doi.org/10.1016/j.jvsvi.2025.100190)
provides a 2025 summary of the mechanisms and cellular participants of neovascularization and fibrinolysis.
The severity of chronic ischemia arising from progression of multi-level arterial occlusive is attenuated by Neovascularization (NV). No matter how clever and refined our surgical/interventional procedures have become, the emphasis needs to change if we are truly committed to achieving durable, low-risk, cost effective solutions for our chronic ischemia patients.
Keep on reading … https://doi.org/10.1002/term.3284
Address Correspondence to:
Darwin Eton MD FACS DFSVS
198 Garvin Hill Rd
Woodstock VT 05091 USA
[email protected]
Cell: 1-857-400-4023
Get your free consultation
- Need guidance and reassurance?
- Talk to a real person from MedClinics!
- Let's find the perfect doctor together.